SAXS (small-angle x-ray scattering) calibrants are used to accurately determine the sample-to-detector distance, which allows one to compute q (reciprocal-space lengths) for the image on an area detector. This document should help you understand how to find and use the various x-ray calibrants available in the laboratory, or make your own.
The essential criteria for any good calibrant are good reproducibility among samples, reasonably strong (intense) scattering, an appropriate length scale, and several orders of diffraction (a well-ordered sample). Broadly speaking, there are two types of calibrants: those that rely on a periodic structure in the calibrant, and those that exploit the geometrical form factor of the calibrant's constituents. These are discussed in turn.
Periodic calibrants are the traditional choice. In the laboratory, we use two types available immediately: silver stearate (d = 48.68 Å) and silver behenate [1,2] (d = 58.376 Å). For larger d-spacings, a common biophysical standard is rat collagen. The lattice constant d is given variously in the literature as 668 Å - 670 Å. (The former number is Eric Eikenberry's value.)
The silver stearate and silver behenate calibrants are easy to prepare. Fill a capillary at least 1 cm tall with the dry powder and seal it. (Flame sealing is easiest in this case, although the powder will burn if you do not leave at least little space). These samples must be stored in light-tight containers since they will slowly photodegrade. One solution is to wrap a test tube in black electrical tape and store the capillary within. To make the specimen to detector distance more precise, place a 0.1 or 0.2 mm filled capillary within a 1.0 or 1.5 mm capillary. Silver stearate and silver behenate are strong scatterers, so there will still be a sufficient signal.
The rat collagen is more difficult, and considerably more gruesome. (Eric Eikenberry is the expert on this protocol). Take a pair of dull wire cutters and snip a fresh rat tail 3/4 of the way through. Strip out the tendons and drop them into a petri dish containing a standard physiological buffer. The buffer should consist of 20 mM phosphate (pH 7.4) and 150 mM sodium chloride. Keep the tendons wet. Once dry, they will not rehydrate fully, leading to an irreproducible length scale in the range 620 Å to 650 Å.
The tendons are sticky threads which can be threaded through an open (acid-washed) capillary with some difficulty. Be sure to add buffer before sealing the capillary. Because this is animal tissue, the sample will only stay fresh a few days.
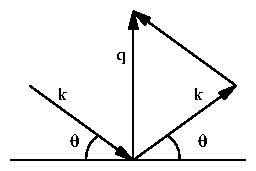
To calibrate an image, it is sufficient to know the specimen-to-phosphor distance z and the x-ray wavelength l.
An x-ray diffraction image from periodic calibrants exhibits Bragg scattering
peaks centered at q given by Bragg's law,
| (1) |
| (2) |
| (3) |


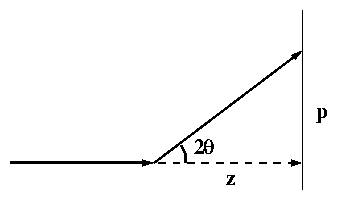
An x-ray diffracted through 2q will strike the detector (phosphor
screen or image plate) at a distance p from the center. As shown in
figure 1(b),
| (4) |
Approximating q » sinq » tanq and
equating q in (2)
and (4),
| (5) |
This procedure for calibrating an image (given z and l) can be
reversed to determine z (given a calibrant of known d and l).
Combining (2) and (5),
| (6) |
To test the validity of the small-angle approximation, consider silver stearate. This has the smallest d-spacing and hence the largest angle per diffraction order. If you used only the n = 10 diffraction peak and the small-angle approximation, you would compute d = 48.4755 Å instead of d = 48.68Å (a relative error of 0.4%). In general, you would use all visible orders n = 1,2, ¼, 10, so the error would be considerably less. Even in this case the error in z will be small. In contrast, the n = 10 diffraction peak for rat collagen produces only a 0.002% relative error by the small-angle approximation.
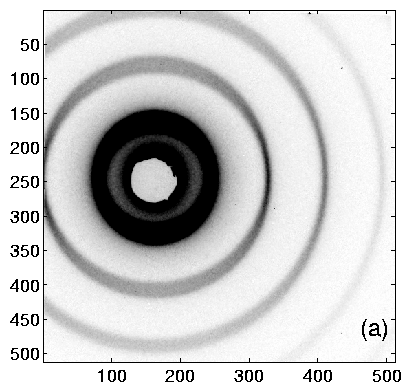
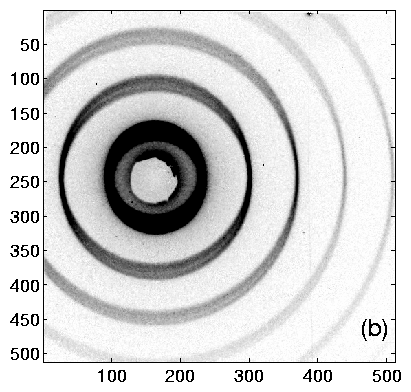
Using TV6, the procedure is as follows. Collect an diffraction image from the calibrant. The ideal image will have many orders of diffraction, with both (left and right) first-order peaks visible. Having at least one order on each side of the center helps to determine the q = 0 point accurately. Example images are shown in figure 2, and integrated profiles in figure 3.
After creating an intensity profile with dens, enter peak. Select the peaks by moving the mouse and entering numbers corresponding to the diffraction order. Press c to calibrate, and d to find the spec-to-phos distance. Enter the calibration length. You can check this calibration by fitting with f and regraphing with g, then showing tick marks with t. Record z, the spec-to-phos distance in pixels. This enables you to recreate the calibration later without repeating all of these steps.
Alernatively, you can do this in Matlab. In TV6, save your data as an rfile; this can be read by Matlab. Assuming the name of your rfile is rfilename.rf:
>> [x,y] = read_rfile('rfilename.rf');
>> qt_slice(x,y)
Then, choose Calibration -> Define calibration and answer the prompts. This defines a calibration by determining z given the l you enter. You can then calibrate this same image by choosing Calibration -> Calibrate or select another image first (File -> Load).
At present, four containers of spherical calibrants are available. First are polystyrene spheres from Duke Scientific (3050A) of radii 25 nm ± 1.0 nm and 1% concentration. Also from Duke Scientific (5020A) are larger spheres of radii 101.5 nm ± 2.2 nm and 10% concentration. Rudolf Sprik generously gave us two vials of silica spheres. The one labelled mEC1 is of radii of 27.8 nm ± 0.4 nm, while the mESiJ3-113 has nominal radii of 136 nm.
The procedure for handling the colloidal suspensions, as given by Duke Scientific:
``For ease of use, these standards are packages in an aqueous suspension. They must be thoroughly dispersed in the bottle to assure statistically consistent samples. Allow the contents to come to room temperature before use. To disperse the particles, gently invert the bottle several times, then immerse in a low power ultrasonic bath (30 seconds). Do not shake the bottle, as the small bubbles formed may introduce statistical artifacts. Before using, be sure no solid material or clumps are visible inside the bottle. Clear the dropper tip by dispensing 2-3 drops into a waste container. Dispense immediately after dispersion using the dropper tip.''
With these precautions, filling a capillary with any solution straightforward. I prefer to flame-seal the tops.
Following
references [3,],
consider a sphere with radius R and uniform electronic density r0
(relative to the surrounding medium),
| (7) |
| (8) |
| (9) |
| (10) |
| (11) |
| (12) |
First, examine the limiting forms of the spherical form factor, F(x).
At low x, F approaches unity.
| ||||||||||||||||||||
| ||||
The average value of the intensity factor F2(x) can be found from
expanding
| ||||||||||||||||||
| |||||||||||||||||||||||||||
The intensity minima occur where F(x) = 0, that is,
x = tanx. For large x, this approaches x = n p+ p/2. So, if
many diffraction minima can be seen, their spacing will yield q R directly.
The intensity maxima occur where dF/dx = 0, or
| (22) |
| n | qR | R = 250Å | R = 278Å | R = 1015Å | R = 1360Å |
| 1 | 5.7635 | 0.2305 | 0.2073 | 0.0568 | 0.0424 |
| 2 | 9.0950 | 0.3638 | 0.3272 | 0.0896 | 0.0669 |
| 3 | 12.3229 | 0.4929 | 0.4433 | 0.1214 | 0.0906 |
| 4 | 15.5146 | 0.6206 | 0.5581 | 0.1529 | 0.1141 |
| 5 | 18.6890 | 0.7476 | 0.6723 | 0.1841 | 0.1374 |
| 6 | 21.8539 | 0.8742 | 0.7861 | 0.2153 | 0.1607 |
| 7 | 25.0128 | 1.0005 | 0.8997 | 0.2464 | 0.1839 |
| 8 | 28.1678 | 1.1267 | 1.0132 | 0.2775 | 0.2071 |
| 9 | 31.3201 | 1.2528 | 1.1266 | 0.3086 | 0.2303 |
| 10 | 34.4705 | 1.3788 | 1.2399 | 0.3396 | 0.2535 |
| n | qR | R = 250Å | R = 278Å | R = 1015Å | R = 1360Å |
| 1 | 5.7635 | 272.54 | 303.07 | 1106.5 | 1482.6 |
| 2 | 9.0950 | 172.71 | 192.05 | 701.20 | 939.54 |
| 3 | 12.3229 | 127.47 | 141.75 | 517.53 | 693.43 |
| 4 | 15.5146 | 101.25 | 112.59 | 411.06 | 550.78 |
| 5 | 18.6890 | 84.049 | 93.463 | 341.24 | 457.23 |
| 6 | 21.8539 | 71.877 | 79.927 | 291.82 | 391.01 |
| 7 | 25.0128 | 62.800 | 69.833 | 254.97 | 341.63 |
| 8 | 28.1678 | 55.766 | 62.011 | 226.41 | 303.36 |
| 9 | 31.3201 | 50.153 | 55.770 | 203.62 | 272.83 |
| 10 | 34.4705 | 45.569 | 50.673 | 185.01 | 247.90 |
With this information, the peak minima or maxima can be fit to determine R. Or if R is known (in the case of a calibrant), then q can be determined. However, one important advantage of spherical calibrants is that the I(q) form has significance, unlike periodic calibrants, which can only provide a single length scale.
If there is any dispersity of radii, this will influence the intensity
spectrum. Assuming a Gaussian distribution centered at
x0 with standard deviation s, the observed intensity is
| (23) |
The simplest calibration method is to determine the q spacing of peak maxima or minima far from the origin, as this value rapidly approaches p/ R as n increases. The next approach is to fit the minima/maxima positions, in analogy to the procedure for periodic calibrants. Here, the peak positions are not linear with q, but can be numerically determined from (22) or table 1.
With TV6, you can fit the intensity maxima, but not the entire lineshape. Begin ``finished'' image. That is it should be dezingered, background-subtracted, intensity-corrected, and distortion-corrected. For example, if your data images are dat1 and dat2 and backgrounds are bak1 and bak2:
>> move im1=dat1:dat2
>> move im2=bak1:bak2
>> move im3=im1-im2
>> move im4=im3!imi
>> move im5=im4!imd
This assumes that you have set the distortion correction images (with setdist) and intensity correction images (with setint).
Display your image with kut=0 (vary scal to taste):
>> disp im5 0 scal
Perform a dens with per-pixel normalization off; this enhances the weaker large-q peaks. Then, enter peak and edit the x-ray wavelength with x. Choose j to enter d-spacings. Pick values from table 2 corresponding to your calibrant. Then, choose c to calibrate and p. Now, TV6 will print z as the spec-to-phos distance in pixels. Unfortunately, it doesn't display a linear fit of d-spacing vs. pixel number, so the quality of the fit cannot be evaluated.
TV6 is now calibrated. Future fits in this TV6 session will use this value for z.
[ Note: as of 2000-01-24, this may no longer be correct. The Matlab code is changing rapidly, and I haven't checked that this sequence of commands is correct. ]
The approach I take here is to fit the entire intensity profile.
To fit these in Matlab, load an rfile as described in section 2.2. To best see the intensity oscillations, choose Options -> Use Circles (off) and Options -> Y-axis: Porod Plot. Typically, the data near q = 0 may be overexposed and the data far from q = 0 will have a lot of noise and weak oscillations. To select only a portion of the data, choose Action -> Crop and select an range in x by clicking and dragging the mouse. (Only the x values are significant; the y-extent of the box is irrelevant.)
Now, choose Estimate and a box labeled qtgui:function will appear. Choose Spherical Calibrants and constant background. After the parameter box appears, you can enter parameters directly, or choose Function -> Estimate again from the main window. At this point, you can choose Function -> Fit and check if the parameters are close by comparing the line shapes. Otherwise, enter new values into the parameter edit boxes and either Action -> Redraw or re-fit.
If the fit is successful, then you know the mean
mean radius in these arbitrary (pixel) units
Rfit, as well as the true radius Rtrue.
We also have
| (24) |
| (25) |
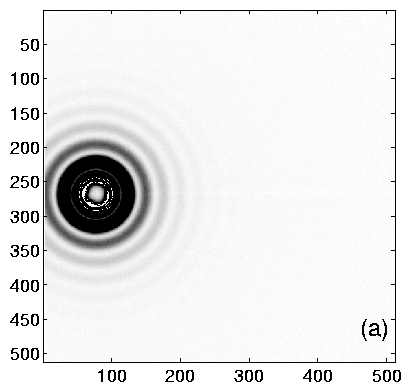
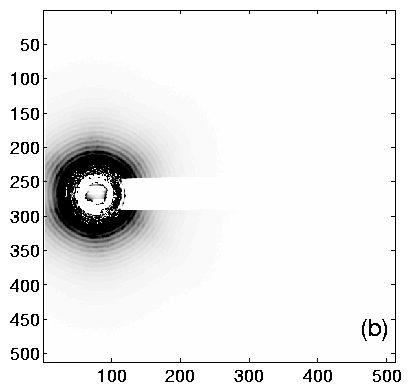


All pre-made capillaries are in the top drawer of the first bench as you enter the wet room. These capillaries are detailed in table 3 below. Also in that drawer are materials for making spherical calibrant capillaries. In the dry box to the left are silver stearate and silver behenate.
| calibrant | length scale (Å) | quantity | keyword |
| Ag-stearate | 48.68 | 6 | ACF Ag-ST 3 ¼7; |
| MWT 3-5-98 | |||
| Ag-behenate | 58.376 ± 0.006 | 5 | ACF Ag-BE 1 ¼5 |
| silica spheres | 278 ± 4 | 6 | ``synthetic rat''; |
| ACF p.41-2 3A ¼ 3E | |||
| silica spheres | 1360 | 5 | ACF p.41-2 4A ¼ 4E |
| polystyrene spheres | 250 ± 10 | 2 | ACF p.58-1 1B; |
| ACF p.131-1 1C | |||
| polystyrene spheres | 1015 ± 22 | 6 | ACF p.58-1 2A; |
| ACF p.41-2 2A ¼ 2E |
Cross calibrations are given in tables 4 and 5. These table were constructed by calibrating with the standard in the leftmost column and calculating the length scale (d-spacing or mean radius) of the other standards.
| Ag-ST | syn-rat | 2A | |
| Ag-ST | 48.68 | 281.8 | 1012 |
| syn-rat | 47.82 | 278 | 994 |
| 2A | 48.81 | 283 | 1015 |
| Ag-stearate ACF #3 | Ag-behenate ACF #5 | |
| Ag-stearate ACF #3 | 48.6799 | 58.3398 |
| Ag-behenate ACF #5 | 48.7099 | 58.3757 |
Thanks to Karen Edler for taking time out of her CHESS run and acquiring so many exposures on the spherical calibrants. This was a tedious job. Thanks also to Krysta Levac for supplying me with rat tails, and sparing me the most gruesome task. Rudolf Sprik kindly gave us the silica spheres, and Lois Pollack lent them to me.
If you notice any errors in this document or have trouble using/making
calibrants, please let me know.
Adam C. Finnefrock
adam@bigbro.biophys.cornell.edu